Crystal structures are all around us. We eat them in the form of salt and sugar and in freezing weather we see them in the forms of snow, ice, and sleet. Water is one of the simplest molecules to form crystals, making it an interesting and fun topic of study. However, don’t let the simplicity of water molecules fool you into thinking the process of snow formation is simple! Water crystals can be exceptionally complex and are the focus of extensive scientific inquiry
First, we need to consider the basis of snow; water. Water is made of two hydrogen atoms attached to a single oxygen through covalent bonds (in which atoms share electrons to create linkages and form molecules). It looks like this:
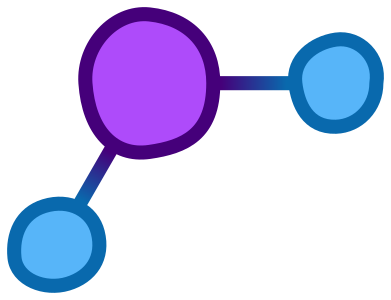
When water is in its gas or liquid state, the molecules bounce around freely, but when colder temperature causes it to solidify, the slightly positive charge of the hydrogen atoms is attracted to the slightly negative charge of the oxygen.This causes the water molecules to form interactions between each other (known as hydrogen bonds) which leads to the development of crystals that generally come together in very specific ways, the most basic of which is shown below.
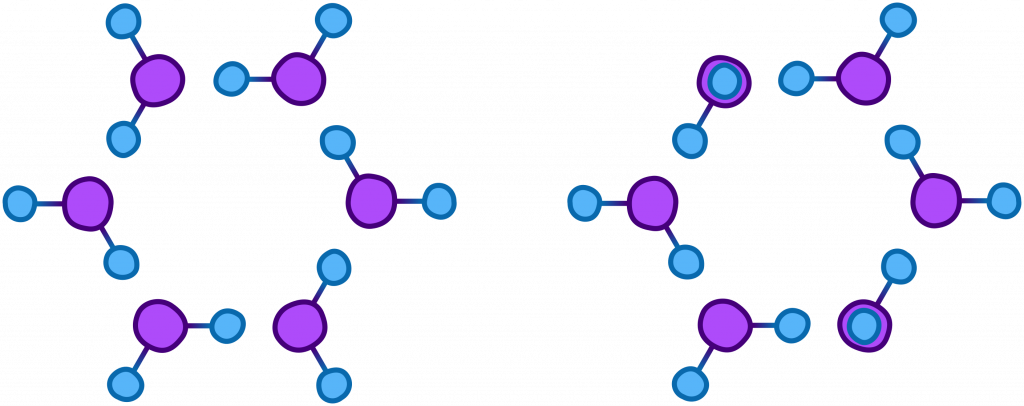
The crystal on the right is a variation in which a few of the hydrogen atoms are pointing up at a 120° angle. They could as easily be pointing in different directions which allows for a diverse variety of snowflakes to be formed
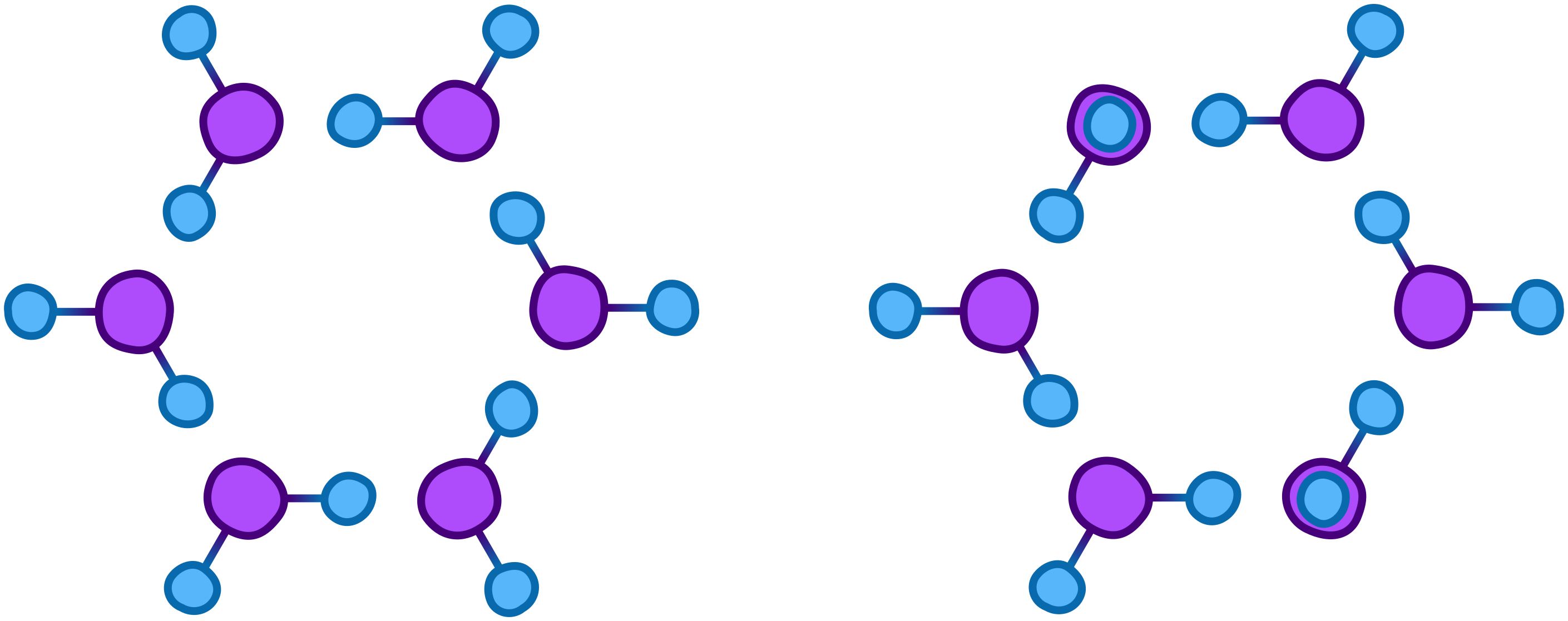
When liquid water freezes, it forms neat crystals and we get ice. But when water vapor deposes, turning directly from a gas to a solid, the molecules don’t have time to form large, clean crystals and instead form snowflakes through a complex additive process. A basic start of a snowflake could look roughly like this:
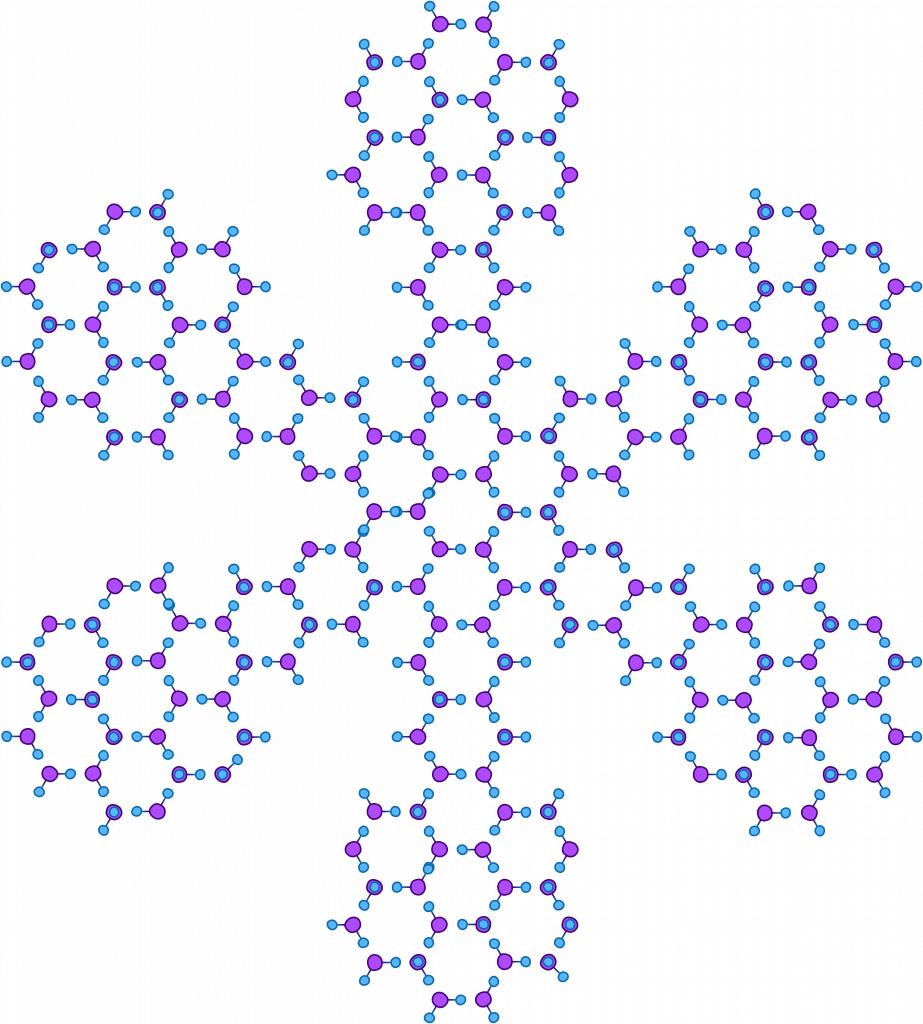
The speed at which snowflakes form combined with other factors including temperature, pressure, and humidity determines the shape, size, structure, and organization of a snowflake. For example, colder temperatures generally form snowflakes that are less elaborate or organized due to the rapid speed of formation.
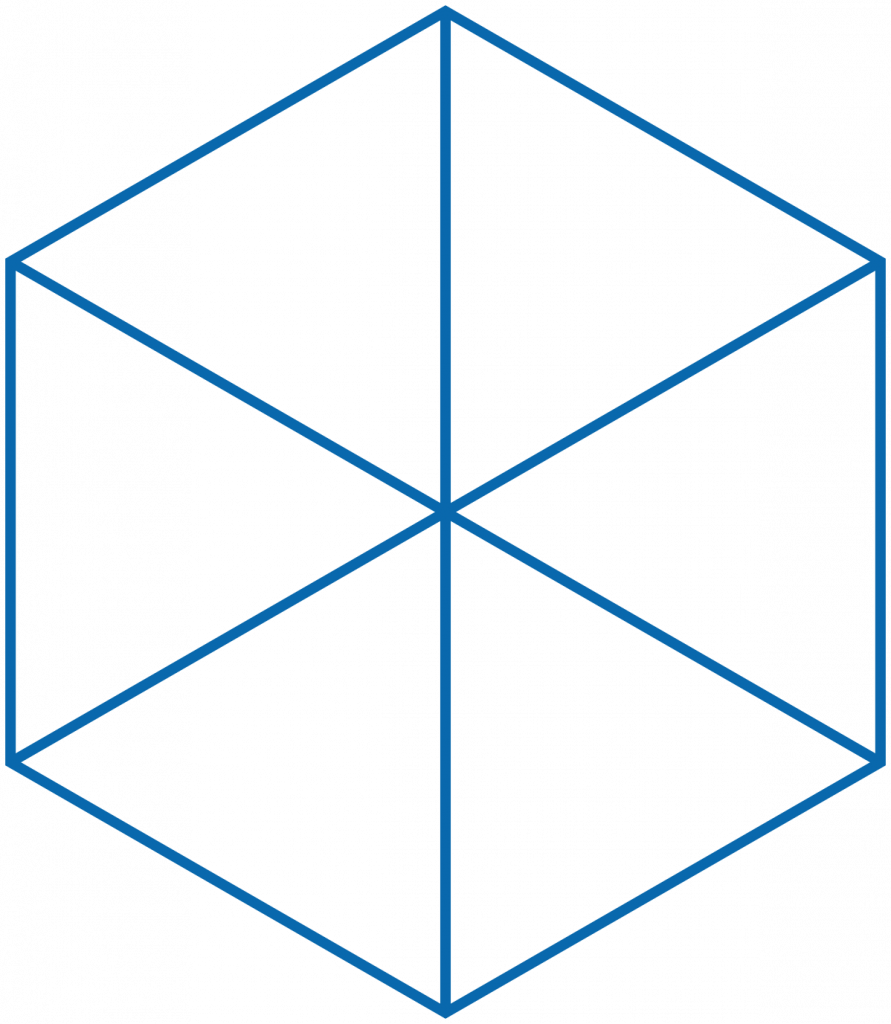
Drawing out all of the individual molecules in a snowflake is a long process and can obscure the larger picture. Thus, they are often simplified as hexagons or star shapes like these. All six arms of the snowflake grow independently, but under near identical conditions as they fall from the sky to the earth’s surface. This permits the formation of complex, symmetrical, and ultimately unique shapes.
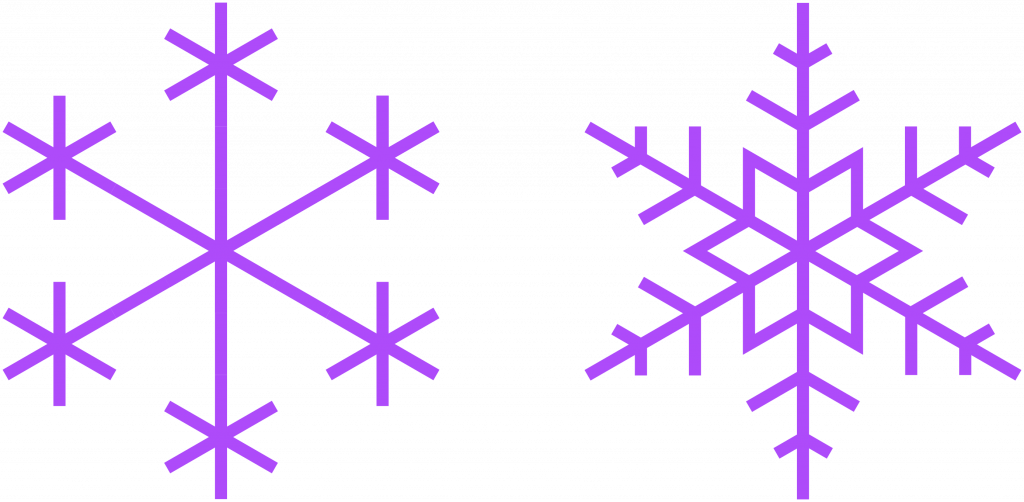
Caltech has an excellent guide on classifying snowflakes and wikipedia has an entire article on snow science that talks about temperatures and classifications. Use those and this simple hexagon guide to draw your own snowflakes.